Randomization with Flexible Block Sizes with randomforge
Friedrich Pahlke
2025-11-26
Source:vignettes/randomforge_flexible_pbr_example.Rmd
randomforge_flexible_pbr_example.RmdIntroduction
This vignette provides a practical example for randomforge. It demonstrates:
- How to define treatment arms
- How to configure project-level randomization
- How do you implement permuted block randomization (PBR) with a fixed starting block size and continuation with variable block sizes
- How to perform quality control of random values
- How to extract and inspect the generated randomization list
Loading the randomforge Package
library(randomforge)
#> randomforge developer version 0.1.0.9046 loadedTrial Setup
Treatment Arms
treatmentArmIds = c("Treatment", "Control")Seed
The seeds in this example will be generated via
random.org
seed <- createSeed()
seed#> [1] 1379678Creating Project and Configuration
randomDataBase <- getRandomDataBase()
randomProject <- getRandomProject(name = "Flexible PBR Example Trial")
randomDataBase$persist(randomProject)
randomConfiguration <- getRandomConfiguration(
randomProject = randomProject,
treatmentArmIds = treatmentArmIds,
seed = seed
)
randomDataBase$persist(randomConfiguration)
ravService <- getRandomAllocationValueService()Initial Randomization Using Fixed Block Size
Define Initial Block Length
initialBlockLength <- 8
randomMethod <- getRandomMethodPBR(
blockSizes = getBlockSizes(treatmentArmIds, initialBlockLength)
)
for (i in 1:initialBlockLength) {
suppressMessages(getNextRandomResult(
randomDataBase, randomProject, randomMethod, ravService
))
}Variable Block Sizes for Remaining Subjects
Define Candidate Block Sizes
blockSizes <- getBlockSizes(treatmentArmIds, c(4, 6))
blockSizeRandomizer <- getRandomBlockSizeRandomizer(
blockSizes = blockSizes,
seed = 2758500)
blockSizeRandomizer$initRandomValues(numberOfBlockSizes = length(blockSizes))Randomize Remaining Subjects
randomMethod <- getRandomMethodPBR(
blockSizes = blockSizes,
fixedBlockDesignEnabled = FALSE,
blockSizeRandomizer = blockSizeRandomizer
)
for (i in 1:(maxNumberOfSubjects - initialBlockLength)) {
suppressMessages(getNextRandomResult(
randomDataBase, randomProject, randomMethod, ravService
))
}Quality Control
Distribution of Used Random Values
plot(ravService)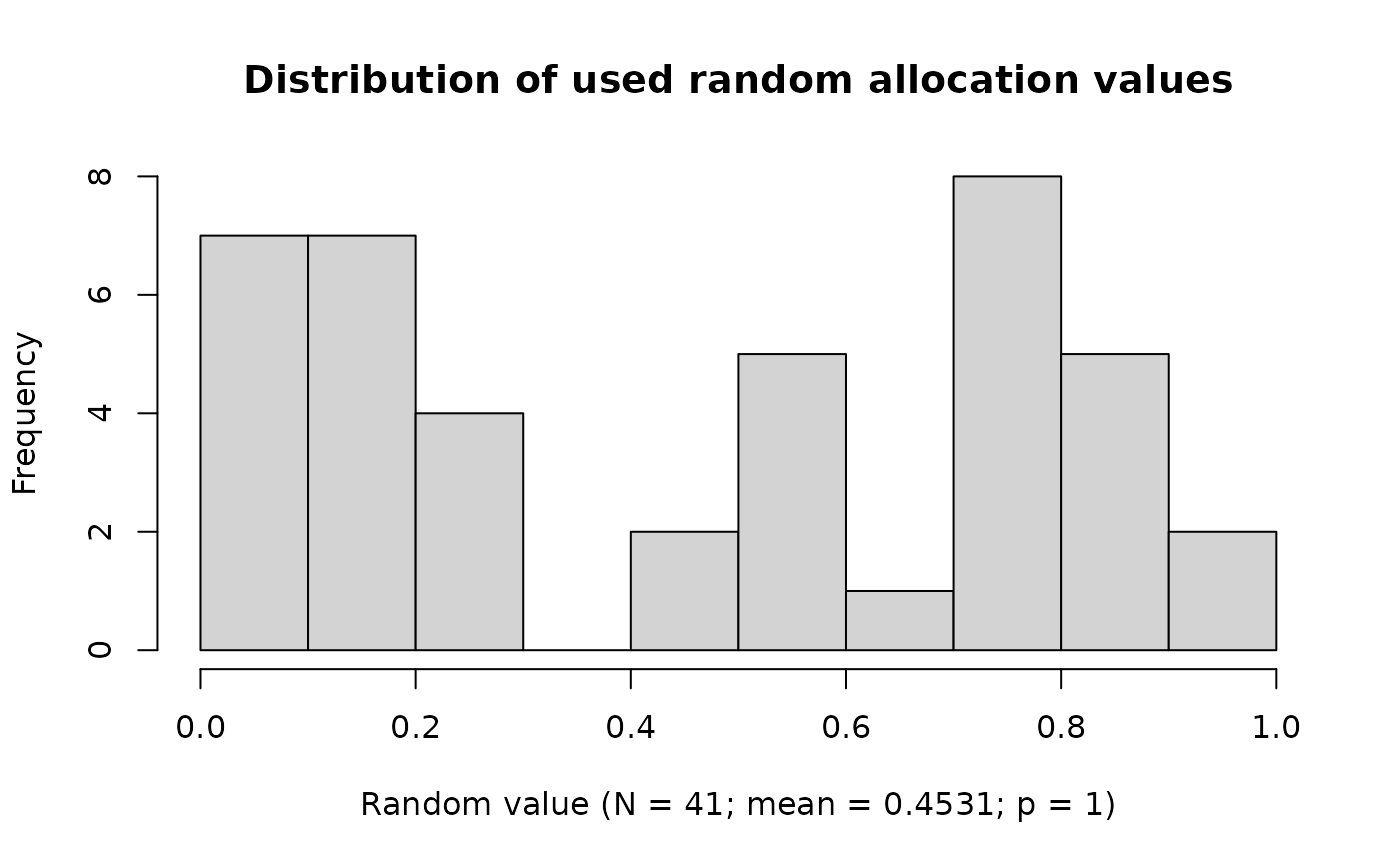
No significant deviation from a uniform distribution is detected (p > 0.05).
Output: Randomization List
randomList <- as.data.frame(randomDataBase)
knitr::kable(randomList)| project | random-number | treatment-arm | status | overall-levels-Treatment | overall-levels-Control | block-wise-levels-Treatment | block-wise-levels-Control | randomization-decision | unique-subject-id |
|---|---|---|---|---|---|---|---|---|---|
| Flexible PBR Example Trial | 1 | Treatment | RANDOMIZED | 1 | 0 | Treatment:1/4 | Control:0/4 | range-set[Treatment=[0,0.5], Control=[0.5,1]; rav=0.0212203289847821] | d8c8ac28-71fa-4057-92d5-de6367c1322d |
| Flexible PBR Example Trial | 2 | Treatment | RANDOMIZED | 2 | 0 | Treatment:2/4 | Control:0/4 | range-set[Treatment=[0,0.5], Control=[0.5,1]; rav=0.1417948291637] | 705e5507-18ea-473e-afba-2390c44b3f18 |
| Flexible PBR Example Trial | 3 | Control | RANDOMIZED | 2 | 1 | Treatment:2/4 | Control:1/4 | range-set[Treatment=[0,0.5], Control=[0.5,1]; rav=0.703004920156673] | 3710dc82-6f09-413e-b7ca-5496b16fda9f |
| Flexible PBR Example Trial | 4 | Control | RANDOMIZED | 2 | 2 | Treatment:2/4 | Control:2/4 | range-set[Treatment=[0,0.5], Control=[0.5,1]; rav=0.525561064947397] | 4c869b36-a77c-4323-9d39-360ddd60669c |
| Flexible PBR Example Trial | 5 | Treatment | RANDOMIZED | 3 | 2 | Treatment:3/4 | Control:2/4 | range-set[Treatment=[0,0.5], Control=[0.5,1]; rav=0.145504052750766] | 8667d6a0-8108-4613-8d9b-ad7b0ba28626 |
| Flexible PBR Example Trial | 6 | Treatment | RANDOMIZED | 4 | 2 | Treatment:4/4 | Control:2/4 | range-set[Treatment=[0,0.5], Control=[0.5,1]; rav=0.408401624765247] | 8c92c5af-91f8-4e53-9cab-538910e009b7 |
| Flexible PBR Example Trial | 7 | Control | RANDOMIZED | 4 | 3 | Treatment:4/4 | Control:3/4 | range-set[Treatment=[0,0], Control=[0,1]; rav=0.526814301963896] | df2e3225-2afa-4775-b579-976ce26a2e7d |
| Flexible PBR Example Trial | 8 | Control | RANDOMIZED | 4 | 4 | Treatment:4/4 | Control:4/4 | range-set[Treatment=[0,0], Control=[0,1]; rav=0.828539446461946] | 9713461a-aa6d-456f-9795-b15771c93969 |
| Flexible PBR Example Trial | 9 | Control | RANDOMIZED | 4 | 5 | Treatment:0/2 | Control:1/2 | range-set[Treatment=[0,0.5], Control=[0.5,1]; rav=0.615094124572352] | 97d824d7-a6ea-44af-8926-e16757ec9cce |
| Flexible PBR Example Trial | 10 | Treatment | RANDOMIZED | 5 | 5 | Treatment:1/2 | Control:1/2 | range-set[Treatment=[0,0.5], Control=[0.5,1]; rav=0.194894318003207] | dbf2d7a5-a55a-4d2a-83ea-76dc6eb4289a |
| Flexible PBR Example Trial | 11 | Treatment | RANDOMIZED | 6 | 5 | Treatment:2/2 | Control:1/2 | range-set[Treatment=[0,0.5], Control=[0.5,1]; rav=0.014120080973953] | b4add5e6-a2b2-458c-a2fc-aea5644f0321 |
| Flexible PBR Example Trial | 12 | Control | RANDOMIZED | 6 | 6 | Treatment:2/2 | Control:2/2 | range-set[Treatment=[0,0], Control=[0,1]; rav=0.180169035680592] | 2f0dccc1-be46-4cb9-b0f1-2bccb95ced45 |
| Flexible PBR Example Trial | 13 | Treatment | RANDOMIZED | 7 | 6 | Treatment:1/3 | Control:0/3 | range-set[Treatment=[0,0.5], Control=[0.5,1]; rav=0.246198229491711] | d65143fb-d9da-4cdb-90bf-d96eaf0741a9 |
| Flexible PBR Example Trial | 14 | Control | RANDOMIZED | 7 | 7 | Treatment:1/3 | Control:1/3 | range-set[Treatment=[0,0.5], Control=[0.5,1]; rav=0.508522406220436] | f09c59af-b573-4a58-b6ae-f0c37065af1b |
| Flexible PBR Example Trial | 15 | Treatment | RANDOMIZED | 8 | 7 | Treatment:2/3 | Control:1/3 | range-set[Treatment=[0,0.5], Control=[0.5,1]; rav=0.000603425083681941] | 440feba7-f93c-4ee9-9c43-9ead56dfe41c |
| Flexible PBR Example Trial | 16 | Control | RANDOMIZED | 8 | 8 | Treatment:2/3 | Control:2/3 | range-set[Treatment=[0,0.5], Control=[0.5,1]; rav=0.867856977041811] | 8f5f460e-3c10-4670-9d02-a9848c5c223a |
| Flexible PBR Example Trial | 17 | Control | RANDOMIZED | 8 | 9 | Treatment:2/3 | Control:3/3 | range-set[Treatment=[0,0.5], Control=[0.5,1]; rav=0.871452651685104] | 8b3cc7ae-fb21-45a9-8640-0689d485fe7d |
| Flexible PBR Example Trial | 18 | Treatment | RANDOMIZED | 9 | 9 | Treatment:3/3 | Control:3/3 | range-set[Treatment=[0,1], Control=[1,1]; rav=0.758590350393206] | c94876be-fa26-4a84-b239-ac9d892d907c |
| Flexible PBR Example Trial | 19 | Treatment | RANDOMIZED | 10 | 9 | Treatment:1/2 | Control:0/2 | range-set[Treatment=[0,0.5], Control=[0.5,1]; rav=0.179464610526338] | 12d50f53-ffa8-42e6-a723-d23c7d44b0ff |
| Flexible PBR Example Trial | 20 | Control | RANDOMIZED | 10 | 10 | Treatment:1/2 | Control:1/2 | range-set[Treatment=[0,0.5], Control=[0.5,1]; rav=0.943183079361916] | d86c27d0-de1f-4d06-b848-1d9e5ee02cdd |
| Flexible PBR Example Trial | 21 | Control | RANDOMIZED | 10 | 11 | Treatment:1/2 | Control:2/2 | range-set[Treatment=[0,0.5], Control=[0.5,1]; rav=0.763430547900498] | 58fefd66-59e0-48ca-86c7-acf3d4e4fd18 |
| Flexible PBR Example Trial | 22 | Treatment | RANDOMIZED | 11 | 11 | Treatment:2/2 | Control:2/2 | range-set[Treatment=[0,1], Control=[1,1]; rav=0.773297929903492] | 3500d8f7-2301-4d57-854b-f9b2c67e58a1 |
| Flexible PBR Example Trial | 23 | Control | RANDOMIZED | 11 | 12 | Treatment:0/2 | Control:1/2 | range-set[Treatment=[0,0.5], Control=[0.5,1]; rav=0.591403880855069] | 83596c0b-c80d-4de4-939a-367d37edbe57 |
| Flexible PBR Example Trial | 24 | Control | RANDOMIZED | 11 | 13 | Treatment:0/2 | Control:2/2 | range-set[Treatment=[0,0.5], Control=[0.5,1]; rav=0.785737928934395] | cf554ae9-4510-4730-8920-48869788b52b |
| Flexible PBR Example Trial | 25 | Treatment | RANDOMIZED | 12 | 13 | Treatment:1/2 | Control:2/2 | range-set[Treatment=[0,1], Control=[1,1]; rav=0.296541688032448] | 75c79aea-bd56-4353-81f3-8c89a4203690 |
| Flexible PBR Example Trial | 26 | Treatment | RANDOMIZED | 13 | 13 | Treatment:2/2 | Control:2/2 | range-set[Treatment=[0,1], Control=[1,1]; rav=0.0567047209478915] | 6ee0eed0-13a8-42ba-b2d8-38b87c3135cf |
| Flexible PBR Example Trial | 27 | Control | RANDOMIZED | 13 | 14 | Treatment:0/3 | Control:1/3 | range-set[Treatment=[0,0.5], Control=[0.5,1]; rav=0.73090074188076] | 2754df2a-d8d4-4822-920e-c27366b99304 |
| Flexible PBR Example Trial | 28 | Treatment | RANDOMIZED | 14 | 14 | Treatment:1/3 | Control:1/3 | range-set[Treatment=[0,0.5], Control=[0.5,1]; rav=0.110916475299746] | b896c3d8-e082-4c0f-861d-14928a2b6ecf |
| Flexible PBR Example Trial | 29 | Control | RANDOMIZED | 14 | 15 | Treatment:1/3 | Control:2/3 | range-set[Treatment=[0,0.5], Control=[0.5,1]; rav=0.887901743175462] | d7b35bbc-3bbe-4e5a-882c-3105bca9710f |
| Flexible PBR Example Trial | 30 | Treatment | RANDOMIZED | 15 | 15 | Treatment:2/3 | Control:2/3 | range-set[Treatment=[0,0.5], Control=[0.5,1]; rav=0.125286859693006] | 2e0e625c-2b86-41ab-b46e-9cd7a2b1b24e |
| Flexible PBR Example Trial | 31 | Control | RANDOMIZED | 15 | 16 | Treatment:2/3 | Control:3/3 | range-set[Treatment=[0,0.5], Control=[0.5,1]; rav=0.727101013064384] | 011d192e-a8a2-4b10-8f18-ecc328e8b5ee |
| Flexible PBR Example Trial | 32 | Treatment | RANDOMIZED | 16 | 16 | Treatment:3/3 | Control:3/3 | range-set[Treatment=[0,1], Control=[1,1]; rav=0.0377451616805047] | c37c48a9-a9c4-4cc9-9654-1408b2f9ecd2 |
| Flexible PBR Example Trial | 33 | Control | RANDOMIZED | 16 | 17 | Treatment:0/2 | Control:1/2 | range-set[Treatment=[0,0.5], Control=[0.5,1]; rav=0.52538527501747] | 6f9642d7-3f76-49dd-aa51-8daa2643c2a7 |
| Flexible PBR Example Trial | 34 | Treatment | RANDOMIZED | 17 | 17 | Treatment:1/2 | Control:1/2 | range-set[Treatment=[0,0.5], Control=[0.5,1]; rav=0.0736770003568381] | 8a5802f7-b923-40c5-8434-4b7f8e73fe83 |
| Flexible PBR Example Trial | 35 | Treatment | RANDOMIZED | 18 | 17 | Treatment:2/2 | Control:1/2 | range-set[Treatment=[0,0.5], Control=[0.5,1]; rav=0.485733987297863] | 6b8d5917-ec03-4749-9b0c-c43729f8caba |
| Flexible PBR Example Trial | 36 | Control | RANDOMIZED | 18 | 18 | Treatment:2/2 | Control:2/2 | range-set[Treatment=[0,0], Control=[0,1]; rav=0.719684938434511] | a2ea2481-ee6e-4b6c-a8af-1703270ab886 |
| Flexible PBR Example Trial | 37 | Control | RANDOMIZED | 18 | 19 | Treatment:0/2 | Control:1/2 | range-set[Treatment=[0,0.5], Control=[0.5,1]; rav=0.965410947334021] | 8f79dc09-8d63-49bd-bd3d-5da611b40a1b |
| Flexible PBR Example Trial | 38 | Control | RANDOMIZED | 18 | 20 | Treatment:0/2 | Control:2/2 | range-set[Treatment=[0,0.5], Control=[0.5,1]; rav=0.812460775719956] | 2c8c34c8-b2d7-467c-adf8-5b9d32eaf311 |
| Flexible PBR Example Trial | 39 | Treatment | RANDOMIZED | 19 | 20 | Treatment:1/2 | Control:2/2 | range-set[Treatment=[0,1], Control=[1,1]; rav=0.205702553037554] | 8d116bc5-5097-485c-8da3-f7da3a2542c1 |
| Flexible PBR Example Trial | 40 | Treatment | RANDOMIZED | 20 | 20 | Treatment:2/2 | Control:2/2 | range-set[Treatment=[0,1], Control=[1,1]; rav=0.0162965569179505] | 5d876423-2257-4154-ac1a-ba104cf1b485 |